HLA Typing and the Fundamentals of Immunology
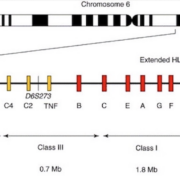
The very essence of HLA typing and its integral role in immunology rests in the highly polymorphic nature of the HLA genes. These genes, responsible for encoding MHC proteins, are key orchestrators of our immune response, a dynamic and complex process designed to protect us from foreign antigens.
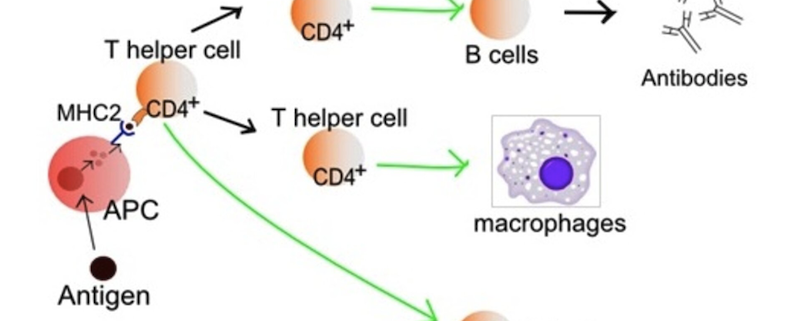
These MHC proteins are categorized into two primary classes: Class I and Class II. Class I MHC molecules are ubiquitous, present on virtually all nucleated cells, whereas Class II MHC molecules are primarily found on antigen-presenting cells (APCs) such as B cells, dendritic cells, and macrophages. Each class plays a unique role in immune response, with Class I MHC molecules presenting endogenous antigens (those originating within the cell), primarily to cytotoxic CD8+ T cells, and Class II MHC molecules presenting exogenous antigens (those taken up by the cell) to CD4+ T helper cells.
The diversity of HLA genes translates into a diversity of MHC molecules, each variant capable of binding to and presenting a distinct set of antigens. The vast repertoire of potential HLA gene combinations thus shapes our individual immune response patterns, determining our susceptibility to different diseases and infections, and influencing our response to treatments such as vaccines.
The Intersection of HLA Typing and Vaccine Development
With the backdrop of this understanding, we begin to grasp how HLA typing has become such a vital tool in vaccine development.
- Personalized Vaccine Design: Individual HLA profiles can guide the creation of customized vaccines. In cancer immunotherapy, a patient’s unique HLA alleles can be studied to predict which neoantigens will bind to the patient’s MHC molecules and elicit a strong immune response. This enables the development of personalized cancer vaccines that stimulate the patient’s immune system to specifically target the cancer cells.
- Comprehending Vaccine Efficacy: The variable efficacy of vaccines across different population groups can be better understood through HLA typing. By recognizing the distinct responses of specific HLA alleles to particular vaccines, researchers can optimize vaccine strategies to enhance efficacy across varied demographics.
- Identification of Novel Vaccine Targets: The study of HLA alleles and their corresponding peptide repertoires can lead to the discovery of novel antigens that could be harnessed as vaccine targets, particularly in the field of infectious diseases.
Advanced HLA Typing Services in Vaccine Research
The future of vaccine research hinges on advancements in HLA typing services. Services offering high-resolution HLA typing employ cutting-edge techniques like Next-Generation Sequencing (NGS) to provide precise HLA typing results. This technology delivers comprehensive insights into an individual’s HLA profile, thereby facilitating the development of personalized vaccines.
Furthermore, HLA typing services can assist in large-scale population studies, helping researchers comprehend the distribution of different HLA types within and across populations, and how this distribution influences the spread and severity of diseases. These insights can guide public health interventions, shaping vaccine distribution strategies to maximize public health benefits.
The Road Ahead
As our understanding of HLA typing continues to evolve, so too will our ability to develop effective and personalized vaccines. The potential applications extend beyond infectious disease and cancer, reaching into areas such as autoimmunity and transplantation.
In the realm of autoimmunity, HLA typing can provide insights into the risk of autoimmune diseases, where certain HLA types are known to be associated with a higher disease risk. This understanding can guide the development of prophylactic interventions, potentially including vaccines, to reduce disease risk in susceptible individuals.
In transplantation, HLA matching between donor and recipient is a critical factor determining transplant success.

 The Sequencing Center
The Sequencing Center
Leave a Reply
Want to join the discussion?Feel free to contribute!