How HLA Typing informs Drug Development
Understanding HLA Binding Specificity to Inform Drug Development
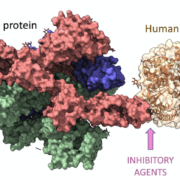
Human leukocyte antigen (HLA) molecules play a critical role in immune responses by presenting peptides to T cells. HLA genes are highly polymorphic, with thousands of HLA alleles known to date. Each HLA allele has a unique peptide binding specificity that is determined by the amino acid sequence lining the antigen binding cleft. Understanding the structural basis of HLA-peptide interactions provides valuable insights that can guide drug development to reduce adverse immune reactions.
HLA Typing to Determine Allelic Variants
The first key step is comprehensive HLA typing to identify allelic variants at the HLA-A, -B, -C, -DRB1, -DQB1, and -DPB1 loci. High-resolution HLA typing, typically by sequencing-based methods, provides allele-level resolution that includes synonymous and non-synonymous substitutions. This discriminates between alleles that differ at the amino acid level, which directly impacts peptide binding[1]. Large population studies have characterized HLA diversity across major ethnic groups to facilitate imputation-based HLA typing from SNP genotyping data[2]. However, direct HLA sequencing is still the gold standard when analyzing HLA associations for drug hypersensitivity reactions.
Structural Analysis of HLA Binding Pockets
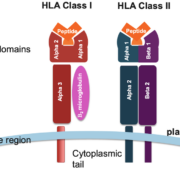
The antigen binding cleft of HLA class I and class II molecules comprise six distinct pockets (A-F) that accommodate peptide side chains at anchor positions. HLA typing identifies which allelic variant of the HLA heavy chain is present. X-ray crystallography of HLA molecules has elucidated the conformation of these pockets and how they interact with peptides[3]. For instance, anchor residues at peptide positions 2 and 9 nestle into pockets B and F of HLA class I molecules. Understanding pocket architecture and chemical specificity provides the framework to predict peptide binding for a given HLA allotype.
In Silico HLA-Peptide Binding Predictions
Leveraging this structural knowledge, in silico methods have been developed to predict peptide binding affinity for specific HLA alleles[4]. These computational tools account for physicochemical properties and contact potentials between peptide side chains and HLA pockets. Machine learning approaches can integrate data from in vitro peptide binding assays to improve predictive accuracy. By screening drug-derived peptide sequences, one could identify potential HLA ligands and high-risk alleles they preferentially bind. This in silico immunogenicity assessment facilitates proactive risk mitigation early in the drug development process[5].
Prospects for HLA-Tailored Therapeutics
Looking ahead, a long-term goal is to develop HLA-tailored peptide-based therapeutics that strategically target binding pockets of high-risk alleles. This could involve modifying anchor residues of immunogenic peptides to ablate binding to off-target HLA variants while preserving specificity for the intended restricting allele. Advanced techniques in protein engineering and deep learning may one day enable such bespoke immunomodulatory agents. Thus, foundational HLA typing and binding studies lay the groundwork for personalized, HLA-directed medicines.
References
[1] Robinson J et al. The IPD and IMGT/HLA database: allele variant databases. Nucleic Acids Res. 2015.
[2] Dilthey A et al. Multi-population classical HLA type imputation. PLoS Comput Biol. 2013.
[3] Illing PT et al. Immune self-reactivity triggered by drug-modified HLA-peptide repertoire. Nature. 2012.
[4] O’Donnell TJ et al. MHCflurry: Open-Source Class I MHC Binding Affinity Prediction. Cell Systems. 2018.
[5] Southwood S et al. In silico immunogenicity assessment for sequences containing unnatural amino acids. Front Dev & Safer Immuno Ther. 2022.
Bassani-Sternberg M et al. Direct identification of clinically relevant neoepitopes presented on native human melanoma tissue by mass spectrometry. Nat Commun. 2016.
Citations:
[1] https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8589423/
[2] https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8500648/
[3] https://www.frontiersin.org/articles/10.3389/fddsv.2022.952326/full
[4] https://www.nature.com/articles/s42256-022-00459-7
[5] https://journals.plos.org/plosone/article?id=10.1371%2Fjournal.pone.0064683

 The Sequencing Center
The Sequencing Center
 The Sequencing Center
The Sequencing Center The Sequencing Center
The Sequencing Center The Sequencing Center
The Sequencing Center
Leave a Reply
Want to join the discussion?Feel free to contribute!