What is the Impact of HLA Typing on Cancer Immunotherapy?
This post explores the influence of Human Leukocyte Antigen (HLA) typing on cancer immunotherapy, detailing its role in developing personalized treatments and improving patient outcomes. We discuss the latest research in the field, including advancements in HLA-based neoantigen discovery, T cell receptor (TCR) engineering, and cancer vaccines.
Setting the Stage
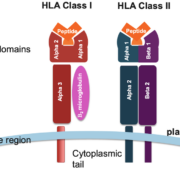
Cancer immunotherapy has revolutionized oncology by harnessing the power of the immune system to target and eliminate cancer cells. HLA typing has played a critical role in the development and optimization of these therapies by providing insights into the interactions between HLA molecules, tumor antigens, and immune cells.
Neoantigen Discovery
Neoantigens, tumor-specific antigens resulting from somatic mutations, have emerged as promising targets for cancer immunotherapy. HLA typing has facilitated the identification of these neoantigens by predicting which HLA alleles can present them to T cells. This knowledge has been instrumental in developing personalized immunotherapies, such as adoptive T cell transfer and neoantigen-based cancer vaccines.
T Cell Receptor Engineering
T cell receptor (TCR) engineering is an innovative approach that involves modifying T cells to recognize and attack specific tumor antigens. HLA typing has contributed to this field by providing insights into HLA-restricted TCR recognition, enabling the design of TCR-engineered T cells tailored to specific HLA alleles.
Cancer Vaccines
Cancer vaccines aim to stimulate the immune system to recognize and attack cancer cells. HLA typing has been crucial in the development of personalized cancer vaccines by identifying tumor antigens presented by specific HLA alleles. Examples include the NeoVax personalized neoantigen vaccine and the RO7198457 cancer vaccine, which are currently undergoing clinical trials.
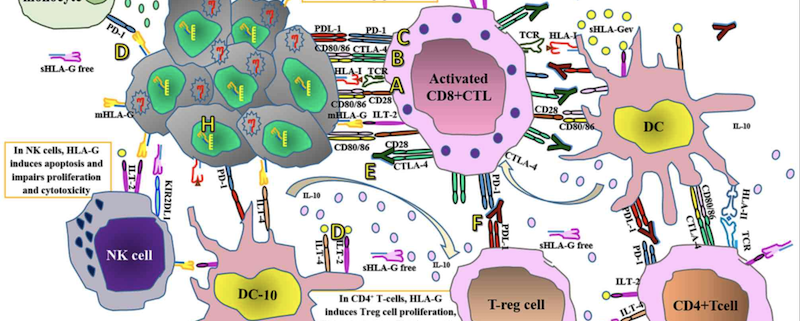
HLA Loss and Immune Evasion
Cancer cells can evade immune surveillance by losing HLA expression, leading to resistance against immunotherapies. HLA typing allows for the detection of HLA loss or downregulation in tumors, enabling the development of novel strategies to overcome immune evasion.
Predicting Response to Immune Checkpoint Inhibitors
Immune checkpoint inhibitors (ICIs) have shown remarkable success in treating various cancers. However, not all patients respond to these therapies. HLA typing has provided insights into the relationship between HLA genotype and ICI response, facilitating patient stratification and the optimization of ICI treatment strategies.
Chimeric Antigen Receptor T-Cell Therapy
Chimeric antigen receptor (CAR) T-cell therapy involves engineering T cells to express a synthetic receptor that recognizes a specific tumor antigen. HLA typing has contributed to the development of HLA-independent CAR T-cell therapies, expanding the applicability of this treatment modality to a broader range of patients.
Monitoring Minimal Residual Disease
HLA typing can be used to monitor minimal residual disease (MRD) in cancer patients by tracking HLA-restricted, tumor-specific T cells in the blood. This information can inform treatment decisions and help detect early signs of cancer recurrence.
Future Directions
As HLA typing technology continues to advance, its impact on cancer immunotherapy is expected to grow. Future developments may include improved neoantigen prediction algorithms, novel strategies to overcome immune evasion, and the integration of HLA typing with other genomic and proteomic data to enhance personalized cancer treatment.
Conclusion
HLA typing has significantly impacted cancer immunotherapy by providing insights into antigen presentation and immune recognition. This knowledge has facilitated the development of personalized immunotherapies, improved patient outcomes, and expanded our understanding of the complex nature of cancer immunotherapy.


 The Sequencing Center
The Sequencing Center
Leave a Reply
Want to join the discussion?Feel free to contribute!